Monday, March 26, 2007
Picture of the day
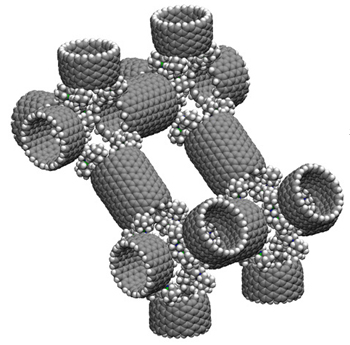
Damian Gregory Allis, Ph.D.: Dative (dipolar) bonds are a potentially valuable form of noncovalent interaction for use in diamondoid and macromolecular nanostructures. These interactions require a lone pair donor, such as the lone pair of nitrogen, and an acceptor, such as the empty sp2 orbital or boron. Boron and nitrogen are both good structural replacements for the C-H fragments found in hydrocarbons (nitrogen because it is isoelectronic with the C-H unit, boron because it can accommodate three covalent bonds to leave the last orbital empty). In this design, carbon nanotubes are functionalized with adamantane-based dative hinges that lock each fragment into place to form the extended network. (Click to see larger version, where: grey = carbon, white = hydrogen, blue = nitrogen, green = boron; left: van der Waals rendering. right: ball-and-stick rendering)
These designs are the result of a collaboration with Dr. Ralph Merkle (www.merkle.com/) into the application of the dative bond in molecular building block approaches for molecular-based materials design.
All images (in this series) are the result of molecular mechanics structure calculations using either Tinker (MM2 parameters) or NAMD (CHARMM). Images were made with VMD. Any inquiries concerning methods, software, or shop talk are directed to http://www.somewhereville.com/.
To see the entire series, visit the Nanotechnology Now Gallery.
Subscribe to:
Post Comments (Atom)










No comments:
Post a Comment